 Ozempic and Mounjaro are two of the “hottest” medications on the market. You don’t have to be a doctor or pharmacist to get to this conclusion; the stock market tells the story. Sales of Ozempic from Novo Nordisk and its rival Mounjaro from Eli Lilly and Company have exploded thanks to Americans’ weight-loss craze. Investors are pouring their money into the two leading manufacturers that develop type 2 diabetes medications because everything points towards even more growth over the next few years.
To illustrate, compared to an increase of 17% in the S&P 500 market index from the end of March through to November 20, 2023, Eli Lilly’s share price almost doubled, and Novo Nordisk went up 41%. There are a few reasons for the exceptional growth in Novo Nordisk and Eli Lilly’s share prices and a good reason why Eli Lilly is outperforming Novo Nordisk so markedly.
To understand what’s going on, it’s important to understand how these two treatments for type 2 diabetes gained so much popularity so quickly. The following few sections will summarize the histories of Ozempic and Mounjaro.
Ozempic and Mounjaro are two of the “hottest” medications on the market. You don’t have to be a doctor or pharmacist to get to this conclusion; the stock market tells the story. Sales of Ozempic from Novo Nordisk and its rival Mounjaro from Eli Lilly and Company have exploded thanks to Americans’ weight-loss craze. Investors are pouring their money into the two leading manufacturers that develop type 2 diabetes medications because everything points towards even more growth over the next few years.
To illustrate, compared to an increase of 17% in the S&P 500 market index from the end of March through to November 20, 2023, Eli Lilly’s share price almost doubled, and Novo Nordisk went up 41%. There are a few reasons for the exceptional growth in Novo Nordisk and Eli Lilly’s share prices and a good reason why Eli Lilly is outperforming Novo Nordisk so markedly.
To understand what’s going on, it’s important to understand how these two treatments for type 2 diabetes gained so much popularity so quickly. The following few sections will summarize the histories of Ozempic and Mounjaro.
How did Ozempic gain its reputation?
Ozempic (generic name: semaglutide) was first marketed as a drug for managing diabetes in adults living with type 2 diabetes who were not responding adequately to controlled diets and for whom the primary medication (Metformin) wasn’t working. Semaglutide is a glucagon-like peptide-1 receptor agonist (GLP-1RA). GLP-1 is a hormone produced in the gut. It is released in response to food, prompting the release of insulin from the pancreas and blocking the release of glucagon. Insulin’s job is to keep blood glucose from rising too high after eating. Glucagon keeps blood glucose levels from dropping too low. It stimulates the conversion of glycogen stored in the liver to glucose, which can be released into the bloodstream. When blood glucose levels fall too low, the pancreas releases more glucagon. The two hormones work together to stabilize blood glucose levels.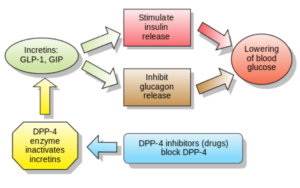
How did Mounjaro gain its reputation?
Mounjaro (generic name tirzepatide) was released in 2021. It combines the same GLP-1RA in semaglutide with a glucose-dependent insulinotropic polypeptide (GIP). Have another look at the top left corner of the diagram above describing how these glucose levels are controlled. GLP-1 and GIP are two incretins that increase the release of insulin. The formulation of tirzepatide provides both GLP-1 and GIP, acting as a backup mechanism in case one doesn’t work adequately and also boosting the total efficiency of each molecule. It’s garnered the term “twincretin,” the trials showing that it provides better A1C benefits than pure GLP-1 without adding any gastrointestinal side effects. Initially, Mounjaro was going head-to-head against Ozempic. The two drugs are similar in that they are both taken as weekly injections, giving them the same profile in terms of the benefit they represent over daily medication. In all probability, once Mounjaro reached the market, the main selling points that pushed its popularity were the growing shortage of Ozempic on the shelves in pharmacies and the marginally better comparative results that came out of the SURPASS-2 trials which tested Mounjaro against semaglutide directly. In SURPASS-2, between 82% and 86% of participants given tirzepatide had achieved an HbA1c level below 7.0%, compared to 79% of those given semaglutide. It is not a dramatic improvement, but it is enough to sway doctors to begin prescribing Mounjaro.How did diabetes medications Mounjaro and Ozempic become weight loss treatments?
What makes diabetes drugs so special when it comes to weight loss? It started with Ozempic back in 2019. As feedback from clinical trials was being analyzed, many of the diabetics enrolled in the trials were reporting an unexpected and very welcome side effect. They were losing substantial amounts of weight, and the more overweight they were, the more they were losing. This led Novo Nordisk to sponsor a full round of testing of Ozempic (STEP 1 trials), examining its ability to induce weight loss. The conclusions drawn from STEP 1 were that for overweight or obese adults, a weekly subcutaneous injection of semaglutide plus moderate diet and exercise was likely to produce substantial, sustained, and clinically relevant weight loss. To say that the publication of these trial results caused a wave of interest among consumers is an understatement. Within weeks, social media platforms were awash with posts from people who were overjoyed by the results of using Ozempic for weight loss. In some cases, leading personalities in the entertainment and information sectors climbed onto the bandwagon to publicize their own experiences with Ozempic, and shelves of pharmacies all over the country were swept clean. It even got to the point where people who had been taking Ozempic for their diabetes for several years were unable to get their prescriptions filled because the production of the drug couldn’t keep up with demand. And then along came Mounjaro! The management at Eli Lilly and Company was well aware of the dramatic impact of semaglutide in this new sector of weight control. So immediately after FDA approval of Mounjaro as a treatment for diabetes in 2021, the company began its own series of tests to see how Mounjaro stacked up as a weight-loss drug. In SURPASS-2 trials, tirzepatide in three different strengths was tested against weekly injections of semaglutide, which is only available in Ozempic in a 1.0 mg dose. Reductions in body weight with tirzepatide were dose-dependent, as shown in the table:
Weight loss |
||
| Semaglutide 1.0 mg | ||
| Mounjaro 5 mg | 16.7 lb (7.6 kg) | 12.6 lb (5.7 kg) |
| Mounjaro 10 mg | 20.5 lb (9.3 kg) | |
| Mounjaro 15 mg | 24.7 lb (11.2 kg) | |
Why must Ozempic and Mounjaro be prescribed “off-label” for losing weight?
Until very recently, both of these diabetes medications were only approved by the FDA as diabetes care treatments for people with type 2 diabetes. Doctors can use their judgments to prescribe either for weight loss, as we have described more fully. However, this affects the cost to consumers because most insurance companies do not reimburse patients for off-label drugs they purchase. Novo Nordisk and Eli Lilly and Company have taken quite different routes to get their respective products into customers’ hands as registered weight-loss solutions.How can you get semaglutide as a prescribed weight-loss medication?
Novo Nordisk brought a higher-dose version of Ozempic onto the market in 2022 with the brand name Wegovy. Wegovy delivers 2.4 mg of semaglutide in a weekly injection. The STEP 1 Trial was launched in 2019, with the final report being published in March 2021. The study’s purpose was to establish whether, and on average, how much weight was gained or lost over thirty months by subjects receiving the semaglutide. Participants were adults who were clinically obese (with a body mass index of 30 or higher) or overweight (BMI between 27 and 30 with at least one weight-related comorbidity) and with a history of at least one self-reported unsuccessful dietary effort to lose weight. Participants were split into two groups, with one control group receiving a placebo and the others receiving a 2.4 mg dose of semaglutide. All subjects were instructed to follow the same routines of a healthy diet and physical exercise, and it was a double-anonymized study, meaning that neither the supervising medical staff nor the participants knew whether it was a placebo or a drug. The results speak for themselves:| Control group (injecting placebo) | Semaglutide (injecting 2.4 mg) | |
| Number of participants at the start | 655 | 1306 |
| Percentage of subjects who lost at least 5% of body mass | 31.5% | 86.4% |
| Percentage of subjects who lost at least 10% of body mass | 12.0% | 69.1% |
| Percentage of subjects who lost 15% or more of body mass | 4.9% | 50.5% |
| Average reduction in waistline | 1.7 in (4.4 cm) | 5.6 in (14.1 cm) |
| Average weight loss | 6.4 lb (2.9 kg) | 35.5 lb (16.1 kg) |