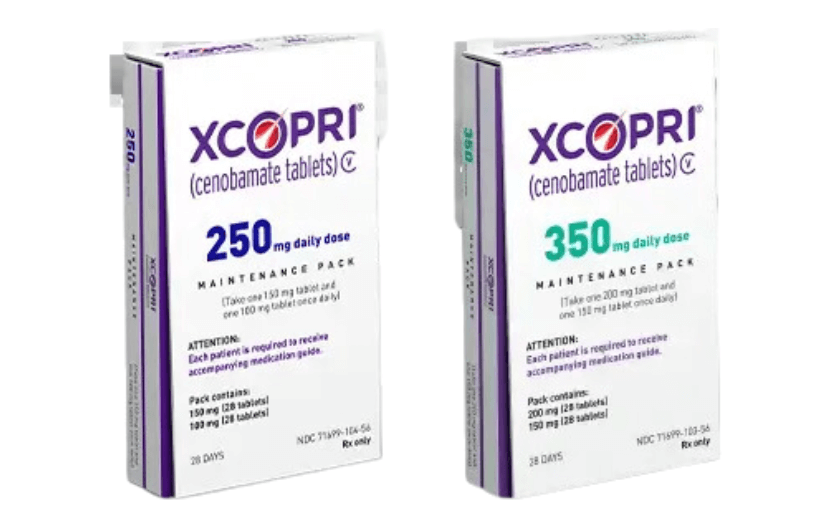
Xcopri (
generic name: cenobamate) is an anticonvulsant medication used to treat
partial-onset seizures in adults. Partial-onset seizures, also known as focal seizures, originate in one area of the brain and can impact motor control, sensory perception, and awareness.
Cenobamate works by modulating the activity of voltage-gated sodium channels and enhancing GABAergic inhibitory currents, which helps to stabilize neuronal firing and prevent seizure propagation. This dual-action mechanism provides
broad-spectrum anti-seizure activity that can be effective even when other treatments have not fully controlled seizure episodes. Xcopri is prescribed for patients whose
partial-onset seizures have not been adequately managed by previous medications. The drug’s efficacy in reducing seizure frequency was demonstrated in clinical trials, showing significant improvement for many patients.
Dosage
It is essential for patients to start taking Xcopri under medical supervision, as the titration schedule must be carefully managed to reduce the risk of side effects. As well, patients are advised not to abruptly stop taking Xcopri without medical guidance, as this could increase the risk of seizure recurrence. A healthcare professional should oversee gradual withdrawal or adjustments.
The dosing for Xcopri starts with a low initial dose that gradually increases over several weeks. Typically, treatment begins at 12.5 mg daily for the first two weeks. The dose is then escalated to 25 mg daily for the next two weeks, followed by increases to 50 mg daily and then 100 mg daily at biweekly intervals. The maximum recommended maintenance dose is generally 200 mg per day, but it can be increased to 400 mg per day based on clinical response and tolerability.
Storage
Store Xcopri in its original containers at room temperature, between 20-25°C (68-77°F), and keep it in a dry place, away from light and moisture.
For more information, read our article on drug storage.
This text is for informational purposes only. Please consult a doctor or pharmacist before using any medication.
Read the information leaflet that comes with the medication.
Most people who use Xcopri do not experience any adverse side effects. Doctors prescribe this medication because they assess the benefits of such treatment outweigh any likely unwanted effects.
Some of the side effects that have been reported include dizziness, drowsiness, and headache. Some patients may experience balance issues or fatigue, particularly during the initial titration phase. Serious side effects requiring immediate medical attention include suicidal thoughts, severe allergic reactions (such as rash or difficulty breathing), and symptoms of a severe skin reaction (Stevens-Johnson syndrome).
Not all side effects are listed here. If these or other unlisted symptoms persist or worsen, consult a healthcare provider or pharmacist.
Xcopri is FDA-approved for the treatment of partial-onset seizures in adults with epilepsy. Partial-onset seizures typically begin in one area of the brain and can result in symptoms ranging from minor twitching to complex physical movements and changes in consciousness. Xcopri has shown promise in reducing the frequency and severity of these seizures, potentially offering patients more stability in daily activities and quality of life.